1. The Problem and Solution approach
Example 1 (Electrical)
1. This Application: The stated problem is to stop ionizing radiation
from affecting transistors in an integrated circuit. Claim 1 reads: an
integrated circuit encapsulated in epoxy resin, characterized in that
said resin includes lead oxide powder.
invention
A
B chanroblesvirtualawlibrary
2. Prior Art A: uses aluminium powder or any other inert powder of good
thermal conductivity in the epoxy resin of an integrated circuit
encapsulation to improve conduction of heat out of the circuit. It says
encapsulation is less than 2 mm thick.
3. Prior Art B: uses a lead box to screen an electrical circuit board
including integrated circuits from ionizing radiation; it says box may
instead be painted with lead oxide.
4.
Prior Art C: shows lead oxide to be inert and to have a better thermal
conductivity than that of aluminium powder.
Using the problem and solution approach the following logical chain of argument results:cralaw:red
1. The Closest Prior Art: Note that although document A has more
features of claim 1 than does document B, document B is chosen as the
closest prior art since it relates to the same problem as the applicant
sets out to solve (the subjective problem).
2. The Distinguishing Features: The difference between claim 1 and document B is that chanroblesvirtualawlibrary

the IC of the claim is encapsulated in epoxy resin including PbO, whereas the IC in document B is enclosed in a box.
3. The Problem: The effect of the epoxy encapsulation compared with the
box is to provide a more compact device, which is also easier to
manufacture. These effects are not stated in either the application nor
in document B, but are readily deduced by comparing I them. Therefore,
the objective problem, derived from the above effect, is to provide a
more compact IC Including a radiation shield.
4. The Solution: Document A discloses how an agent (in this case a
thermal one) may be provided in an lC in a compact manner, i.e. by
incorporating it in an epoxy encapsulation of the IC.
5. Conclusion: Therefore, the skilled person, looking for the problem
stated by the applicant (the subjective problem), would read document B
for a solution, and adopt this as a first step. He would then realize
that the resulting device is rather bulky, and would Seek a solution to
the second problem (the objective problem), and find its solution in
Document A. Since both problems and solutions are disclosed in the
prior art, no inventive step is involved in the IC of claim 1 of the
application.
The same result is obtained by using another approach, as follows:cralaw:red
A skilled person, using the IC of document A, would realize that he
could make it more efficient by replacing the aluminium powder by PbO,
according to the teaching of document C. He would thus make the IC of
claim 1 of the application. By chance, however, he would achieve an
extra and unexpected effect, that of blocking ionizing radiation.
However, this is a bonus effect, not able to support inventive
activity, since the prior art suggests a combination of documents A and
C. A one-way street situation is present since only solution to
improving the thermal conductivity is suggested in the prior art.
Example 2 (Mechanical)
1. Application: This concerns a dining table. The description describes
a prior art table having four legs. The problem with this table is that
it wobbles on an uneven surface. The problem is set out as follows:
"The object of the invention is to provide a dining table that does not
wobble on an uneven surface".
Claim 1 reads: Dining table having a top surface supported by only
three legs so that the center of gravity of the table is located
between the three legs.
2. Prior Art 1: This shows a dining table having four legs, it does not
mention the wobbling problem, but is concerned with heat resistance of
the top surface, to prevent damage by hot pots, etc.
3. Prior Art 2: This shows a dining table having four legs, it does
mention the wobbling problem, and the solution disclosed is to make one
leg telescopic.
4. Prior Art 3: This shows a stool for milking cows, having three legs. It does not mention a wobbling problem.
Using the problem and solution approach the following logical chain of argument results:cralaw:red
1. The Closest Prior Art: This is document 2 which concerns the same
subject-matter as the application and also the same problem, wobbling.
Note that the problem stated by the applicant is the subjective
problem, the wobbling problem in a dinning table having already been
solved (in document 2).
2. The Distinguishing Features: The difference between claim 1 and document 2 is that the table of claim 1 has three legs.
3. The Problem: The effect of the three legs of the invention is that
it is a simpler solution to the solution of a telescopic leg.
Therefore, the objective problem, derived from the above effect, is to
provide a simpler solution to the wobbling problem.
4. The Solution: Document 3 shows that this simple solution was known
in the prior art and is not inventive. Although this document does not
mention anything about wobbling, the skilled person knows that milking
stools are for use on uneven surfaces, and the use of three legs is to
counter wobbling, so this document implicitly discloses the present
solution to the present objective problem.
5. Conclusion: Therefore, the skilled person, looking for the problem
stated by the applicant (the subjective problem), would read document 2
for a solution, and adopt this as a first step. He would then realise
that the resulting device is complicated, and would Seek a solution to
the second problem (the objective problem), and find its solution in
Document 3. Since both problems and solutions are disclosed in the
prior art, no inventive step is involved in the table of claim 1 of the
application.
Example 3 (Chemical)
1. The Application: Claim 1 relates to a reactor for manufacturing urea
in which the reactor walls are made of plates of a corrosion-resistant,
hardened alloy of lead and antimony, wherein the hardened alloy is
prepared by quenching a molten homogeneous mixture of the alloy metals
directly after casting, and rolling the alloy after quenching, the
joints between the plates being made of unhardened alloy of the same
composition.
The problem was to produce urea which is not contaminated by corrosion products.
2. Prior Art Document 1: relates to the preparation of lead antimony
alloys having a lead content of at least 80% by weight and which are
prepared by mixing the molten metals and allowing the cast alloy
mixture to solidify, followed by further processing to form plates.
3. Prior Art Document 2: discloses lead antimony alloys obtained by the method disclosed in claim 1 of the application.
4. Prior Art document 3: discloses the preparation of a pure urea in a
corrosion-resistant reactor wherein the reactor walls are made of
rolled plate of a homogeneous lead antimony alloy. The joints between
the plates are made of pure lead.
Using the pproblem and solution approach the following logical chain of argument results:cralaw:red
1. The closest prior art is document 3 since it deals with the same
problem as the application, namely the production of the corrosive
chemical urea. The solution found is the same as in the application.
2. The difference is that the joints between the plates are filled with lead instead of the same alloy composition.
3. The objective problem was to produce a reactor having a still further improved corrosion resistance.
4. The solution was to use the alloy plates of document 2 and to use the same alloy for the joints.
5. Conclusion: It could be argued that the skilled worker would look to
document 2 to find out how to form an alloy plate suitable for use
according to document 3. However, neither of these documents addresses
the particular problem of avoiding the problem of contact corrosion at
the point of contact between the alloy plates and joints. The advantage
of lack of corrosion is found in the higher purity urea which is
produced. Therefore, the problem was NOT solved in an obvious way and
the application involves an inventive step.
2. Patentability and industrial applicability chanroblesvirtualawlibrary
2.1 Examples of medical claims chanroblesvirtualawlibrary
Example 1
The Claim Reads: Mammography apparatus adapted to receive signals
representing tissue parameters and having a computer, characterized in
that the computer evaluates the radiation dose (D) according to the
formula chanroblesvirtualawlibrary
D= d x l x T x k
where d is the thickness of the tissue, I is the tube current, T is the exposure time, and k is a calibration factor. chanroblesvirtualawlibrary
This claim is allowable since it is not a diagnostic method or a
treatment. Nor is it a purely mathematical method since the numbers
involved in the formula are not abstract numbers, they clearly
represent physical values. Also, the formula calculates the radiation
dose for a patient, i.e. it serves a technical purpose. chanroblesvirtualawlibrary
Even if the only inventive step is in the mathematical evaluation of D,
the apparatus as a whole is technical. Here, a distinction should made
between the "idea underlying the invention", which in the above case is
a mathematical method, and the "contribution that the invention makes
to the art", which is a better dosing of the radiation, and is clearly
technical.
Example 2
The claim reads "A method of measuring blood flow in animal tissue,
comprising injecting radioactive micro spheres into the blood of the
animal, sacrificing the animal, and counting the number of micro
spheres in a given sample of tissue." chanroblesvirtualawlibrary
The above claim includes the step of injecting micro spheres into the
body, and killing the animal, so that "blood is spilt" (See the
definition of surgery in Chapter IV, 4.3).
Nevertheless, the above claim is allowable since the purpose of the
claim is not to heal the animal, there is the opposite step of killing
the animal. This method is for experimental purposes and not concerned
with the health of the animal.
Example 3
The claim reads "A method of making an X-ray image of a living body,
comprising placing the body between source providing a fan-shaped X-ray
beam and a detector consisting of a two-dimensional array of detector
elements, characterized in that a slit scans across the fan-shaped
X-ray beam and corresponding detector elements are simultaneously
energized." chanroblesvirtualawlibrary
This claim is allowable since it does not result by itself in the
diagnosis, even though it serves to provide interim results. For such a
method to be excluded as a diagnostic method all three of the following
conditions must be satisfied: chanroblesvirtualawlibrary
i) The result must immediately provide a diagnosis and not merely interim results.
ii) The method can only be used by a medical practitioner. In the
above case the method can be used by a laboratory technician, a doctor
is not necessary.
iii) Both the examination and the establishing of symptoms must
be carried out on the living body. In this case only the examination is
carried out (by a laboratory technician) on the living body, the actual
diagnosis is done remotely by the doctor. chanroblesvirtualawlibrary
Moreover, the object of the invention is to provide a sharper X-ray
image so the technical problem in the application is clearly technical,
as is the solution. chanroblesvirtualawlibrary
Example 4
The claim reads "A method of improving bodily appearance of a mammal
which comprises orally administering naltrexone in a dosage effective
to reduce appetite, and repeating the dosage until a cosmetically
beneficial loss of weight has occurred." chanroblesvirtualawlibrary
The wording of the claim clearly directs the method to a cosmetic
treatment ("improving bodily appearance", "cosmetically beneficial loss
of weight"). Moreover, a loss of weight is not inevitably linked to an
increase of health, so this claim is allowable. chanroblesvirtualawlibrary
Example 5
The claim reads "A method of cleaning plaque from human teeth by
applying thereto a non-oxidizing aqueous composition consisting of
lanthanum in the form of a water-soluble salt."
Although the removal of plaque from teeth improves their appearance and
is cosmetic, there inevitably follows improved health since plaque
causes tooth decay, so a prophylactic treatment is implicit in this
method, and it is not allowable. chanroblesvirtualawlibrary
Further examples are given in VII Examples, Part 5: "Chemical Problems". chanroblesvirtualawlibrary
2.2 Examples of computer-related inventions chanroblesvirtualawlibrary
Example 1
See example 1 above in examples of medical claims.
Example 2
Claim 1 reads "A data processing system having a plurality of
interconnected data processors in a network, and a plurality of nodes
containing input-output means, means at each node to process a
transaction request and executing a transaction associated with the
request, characterized by means for determining if a transaction
requires another processor, and generating a further transaction
request and transmitting it to the other processor for execution".
Remarks: This claim looks like a business transaction since it is basically a system in
which, for example, a customer inputs a request into a keyboard for
drawing money out of a cash machine. However, the system is technical
subject-matter since it relates to how computer operation is affected,
i.e. two or more computers may be connected together to complete the
transaction. Therefore, this system is patentable.
Example 3
Claim 1 reads "X-ray apparatus having several X-ray tubes,
characterized by a computer which stores tube rating data and uses this
to set exposure parameters for each tube, such that optimum exposure is
obtained for each tube without overloading of anyone of them". chanroblesvirtualawlibrary
Remarks: The apparatus includes the known combination of an X-ray
apparatus and a computer which stores tube data and software for
operating X-ray tubes. However, the new feature is the program that
supervises the operation of the X-ray tubes so as to optimize
performance of each tube as it ages. This is a technical result so the
claim is patentable.
Example 4
Claim 1 reads "A system for automatically abstracting documents and
storing the result, comprising a dictionary memory for storing commonly
used words, means for reading the document and comparing the words in
it with the commonly used words stored, and selecting only those words
not commonly used, and storing the selected words", chanroblesvirtualawlibrary
Remarks: There is no technical result produced here, only a list of
selected words, which is in the nature of intellectual activity only,
so this claim is not patentable. chanroblesvirtualawlibrary
In all the above examples the only difference from the prior art lay in
the computer programs. If the overall result is technical then the
claim is considered allowable. chanroblesvirtualawlibrary
2.3 Other examples chanroblesvirtualawlibrary
Example 1
Claim 1 reads "An electrical signal for application to the deflection
plates of a particle analyzer, comprising a pulse having a plurality of
exponentially falling portions of decreasing exponential function
joined together to form a smooth overall shape. chanroblesvirtualawlibrary
Firstly, it is noted that the claim is a device claim but it relates to
an intangible entity, i.e. an electrical signal. Secondly, it is a
transient entity, since the signal is a pulse, i.e. it is generated
only at the instance it is needed. However, both the above points are
not a bar to patentability, since the signal has a technical character,
it enables the separation of charged particles in a particular manner,
and the claim is allowable.
Example 2
A claim reads "Device for healing living bodies by improving cosmic
harmony with the ("environment, the device comprising a plastics base
and circular wires of different noble metals arranged concentrically on
the plastics base". chanroblesvirtualawlibrary
In such a case an objection should be raised that there is a doubt that
there is a direct relationship between the technical problem and Its
solution. The stated technical problem is that bodies should be healed
"by improving cosmic harmony", but there is a serious doubt that the
stated construction can cause any healing, i.e. that it really is a
technical solution. The applicant should be requested to submit his
invention to a neutral authority or government agency to carry out
tests proving that the device has healing effects. It is not sufficient
that the applicant submits affidavits from private people or doctors or
clinics. Only if these tests prove that the device works as stated by
the applicant can a patent be granted. chanroblesvirtualawlibrary
Example 3
The claim reads: "A method of selecting the correct dosage of a drug
for a patient, comprising measuring the length of the patient's body
from head to foot using a tape having markings along its length, the
markings having indications of the correct drug dosage, and using the
indication appropriate to a given patient to regulate the dosage."
In this case the indications on the tape is a presentation of
information, i.e. excluded from patentability. Thus, the claim contains
a mixture of technical and non-technical features. In order to decide
whether the invention as a whole provides a technical contribution to
the prior art, the prior art must first be considered. This is a tape
having markings along its length, the markings having indications of
the amount of food to be given to a child.
To argue, at this stage, that the only difference between the known
tape and the claimed tape is the information that the markings give (in
one case the drug dosage and in the other case the amount of food), so
the invention is merely a presentation of information, would be wrong,
since the argument does not adequately evaluate the relationship
between the markings and the tape the carries the markings. In other
words it does not take the teaching of the claim as a whole. chanroblesvirtualawlibrary
The new markings make the tape into a new gauge for directly measuring
dosage. It is like a pressure gauge in which the markings of pressure
are replaced by markings that make the gauge suitable for use as an
altimeter. The claim is, therefore, patentable.
Further examples are given below in VII Examples, Part 5 "Chemical Problems".
3. Clarity
Example 1
The claim reads "A device for draining fluids from the stomach,
comprising an inert plastics tube having a smooth outer wall and a
rounded leading end having an aperture, and electrodes embedded in the
outer wall along its length, characterized in that the length of the
tube is such that when it is inserted into the mouth and down into the
esophagus, it reaches the stomach".
Comments: The characterizing feature is indeterminate and the scope of
the claim isunclear. The distance between the mouth and the stomach
depends on the age, sex,racial type, etc. of a person, so it is not
well defined. Moreover, the claim does not limit the device for use
with humans, it could be used with giraffes, in which case the possible
range of lengths is enormous and the claim becomes even more unclear.
Example 2
The claim reads "Apparatus for receiving a credit card and reading it,
comprising a housing for insertion in a recess in a wall and having a
front opening having a size for receiving the card, the front wall of
the housing also including a keyboard for keying in passwords, numbers,
etc., and the inside of the housing including a microprocessor
…….”chanroblesvirtualawlibrary
Comments: The claim is directed to apparatus for reading credit cards,
and does notinclude the card itself. However, one feature of the claim
(size of the front opening) is defined with respect to the card. This
is allowable since credit cards have standard sizes. The situation
might be different if the opening were to be defined with respect to a
coin, for example, since coins have different shapes and sizes.
Example 3
The claim reads "A bracket for hanging a picture, comprising a hook
having two projections, wherein the bracket is secured in an opening in
a wall by rotating it through a given angle, inserting one projection
into the opening, rotating it back to the original orientation, and
lowering it".
Comments: The claim attempts to define the constructional features by
reference tomanner in which the bracket is secured to the opening in
the wall, and is not clear.
Example 4
The Claim reads "Apparatus for recording on or reproducing from a laser
disk, wherein a semiconductor laser beam intensity is varied between a
high value for recording and a low value for reproducing, characterized
in that the laser wavelength is kept constant as the intensity is
varied".
Comments: The claim is unclear since it sets out the desired result
rather than technical features that cause the result. It would be
necessary to define those parts of the apparatus that detect wavelength
variations and a feedback loop for maintaining the wavelength constant.
Example 5
The Claim reads "Apparatus for recording on or reproducing from a laser
disk, wherein a semiconductor laser beam intensity is varied between a
high value for recording and a low value for reproducing, characterized
in that the laser wavelength is kept constant as the intensity is
varied, or the variation from high to low value is performed by a high
speed switch, or the laser temperature is kept constant as the
intensity changes".
Comments: The different options in the claim are not fairly exchangeable and lead to confusion as to the scope of the claim.
Example 6
The Claim reads "Apparatus for pacing the heart comprising a pulse
generator, wherein natural heart beats are detected, and if one or more
natural heartbeats is missing, a detector measures blood pressure and
if this is below a given threshold a stimulating pulse is issued".
Comments: This claim not only defines the desired result instead of
technical features that give the result, but is further objectionable
since it relates to apparatus but sets out method steps. so its
category is also unclear.
4. Unity of Invention
The following examples illustrate how to apply the principle of unity of invention in particular cases.
4.1 Claims in different categories
Example 1
Claims:cralaw:red
1. A process for manufacturing chemical substance X.
2. Substance X.
3. The use of substance X as an insecticide.
Prior art:cralaw:red
(i) Situation 1: Substance X is novel and inventive.
(ii) Situation 2: The search has revealed that the substance X lacks novelty with regard to the state of the art.
Comments:cralaw:red
(i) Situation 1: The feature common to all of claims 1 to 3 is the
substance X. This common feature is a special technical feature, since
it makes a contribution over the prior art. Thus, there is a special
technical feature common to all the claims. Consequently unity exists
between the inventions defined in claims 1, 2 and 3.
(ii) Situation 2: Claim 2 fails for lack of novelty and must be
deleted. The feature common to the remaining claims 1 and 3 is the
substance X. However, the substance X is not a special technical
feature, because it makes no contribution over the prior art. Thus,
there is no special technical feature common to claims 1 and 3.
Consequently there is lack of unity "a posteriori" between claims 1 and
3.
Example 2
Claims:cralaw:red
1. A process of manufacture comprising steps A and B.
2. Apparatus specifically designed for carrying out step A.
3. Apparatus specifically designed for carrying out step B.
Prior Art:cralaw:red
The search has revealed no relevant prior art document related to the process of claim 1.
Comments: Steps A and B respectively are two special technical features
which define contributions over the prior art. Unity exists between
claims 1 and 2, or between claims 1 and 3. There is no unity, however,
between claims 2 and 3, since neither the same nor a corresponding
special technical feature is common to both claims 2 and 3, and one of
these claims must be cancelled.
Example 3
Claims:cralaw:red
1. A process for painting an article in which the paint contains a rust
inhibiting substance X, said process including the steps of atomizing
the paint using compressed air, electrostatically charging the atomized
paint using an electrode arrangement A, and directing the paint onto
the article.
2. A paint containing substance X.
3. A paint spraying apparatus including electrode arrangement A.
Prior Art:cralaw:red
The paint containing substance X is new and inventive. The electrode
arrangement A is also new and inventive. However, it is known to paint
an article by atomizing the paint using compressed air, and
electrostatically charging the atomized paint, and directing the paint
onto the article.
Comments: The special technical features of claim 1 are (i) to use a
paint containing substance X, and (ii) to use the electrode arrangement
A. Special technical feature (i) is to be found in claim 2, such that
there is a technical relationship between the inventions of, and thus
unity between, claims 1 and 2. Special technical feature (ii) is to be
found in claim 3, such that there is a technical relationship between
the inventions of, and thus unity between, claims 1 and 3. The special
technical feature (i) of claim 2 is neither the same nor corresponding
to the special technical feature (ii) of claim 3. Thus, there is lack
of unity between claims 2 and 3 and one of these claims must be
cancelled. chanroblesvirtualawlibrary
Example 4
Claims:cralaw:red
1. Use of a family of compounds represented by the general formula X as insecticides.
2. Compound X1 belonging to family X.
Prior Art:cralaw:red
Compound X1 is novel and inventive. Certain other members of the family
of compounds X are, however, comprised in the prior art. There is no
disclosure of an insecticidal activity for these known compounds.
Comments: The special technical feature in claim 1 is the insecticidal
use of the structure. Provided that X1 has an insecticidal activity
unity is present. chanroblesvirtualawlibrary
Example 5
Claims:cralaw:red
1. A process for treating textiles comprising spraying the material
with a particular coating composition under special conditions (e.g.,
as to temperature, irradiation).
2. A textile material coated according to the process of claim 1.
3. A spraying machine for use in the process of claim 1 and
characterized by a new nozzle arrangement providing a better
distribution of the composition being sprayed.
Prior Art:cralaw:red
A prior art document discloses a process for treating textiles with a coating. The process according to claim 1 is novel.
Comments: In. respect of the prior art, the textile material of claim 2
exhibits unexpected properties. In claim 1 the use of special process
conditions corresponding to what is made necessary by the choice of the
particular coating is the special technical feature which makes the
contribution over the prior art. This special technical feature
corresponds to the limitation of the textile product with its
unexpected properties according to claim 2. Thus, unity exists between
claims 1 and 2. The spraying machine in claim 3 does not involve any
feature corresponding to the above identified special technical
feature. Therefore unity does not exist between claims 1 and 3, nor
between claims 2 and 3, so Claim 3 should be cancelled.
Example 6
Claims:cralaw:red
1. A chair (A), coated with layer X.
2. A chair according to claim 1, made of wood.
3. A process for distributing the layer X.
Prior art:cralaw:red
A prior art document discloses chair (A); a further document discloses
layer X, but not in the field of furniture. The application of layer X
to a chair (A) provides a special effect, e.g. protective qualities,
for wooden furniture, not produced for other objects.
Comments: The combination of the features A and X in claim 1 is not
obvious, since a special effect is achieved. Thus said combination is a
special technical feature making a contribution over the prior art. The
same special technical feature IS automatically contained in the
dependent claim 2. Therefore unity exists between claims 1 and 2. There
is no unity, however, between claims 1 and 3, since claim 3 does not
include said special technical feature, i.e. the combination of the
features A and X.
Example 7
Claims:cralaw:red
1. A fuel burner with tangential fuel inlets into a mixing chamber.
2. A process for making a fuel burner including the step of forming tangential fuel inlets into a mixing chamber.
3. A process for making a fuel burner, including a casting step.
4. An apparatus for carrying out a process for making a fuel burner,
including feature X resulting in the formation of tangential fuel
inlets.
5. An apparatus for carrying out a process for making a fuel burner, including a protective housing.
6. A process of manufacturing carbon black, including the step of
tangentially introducing fuel into a mixing chamber of a fuel burner.
Prior Art:cralaw:red
A prior art document discloses a fuel burner with non-tangential fuel
inlets and a mixing chamber. Tangential fuel inlets are neither known
nor obvious from the relevant prior art.
Comments: The feature of the tangential fuel inlets in claim I is the
special technical feature which makes the contribution over the prior
art. This special technical feature is common to claims 1, 2, 4 and 6.
Thus, unity exists between these claims. Claims 3 and 5 do not include
the same or a corresponding special technical feature, so that they
lack unity with claims 1, 2, 4 and 6, and with one another.
Example 8
Claims:cralaw:red
1. A high corrosion resistant and high strength ferritic stainless
steel strip consisting of, in percent by weight: Ni = 2 -5; Cr = 15
-19; Mo = 1 -2; and the balance Fe, having a thickness of between 0.5
and 2.0 mm and a 0.2% yield strength in excess of 50 kg/mm2.
2. A method of producing a high corrosion resistant and high strength
ferritic stainless r~ steel strip consisting of, in percent by weight:
Ni = 2 -5; Cr = 15 -19; Mo = 1 -2; and the balance Fe, comprising the
steps of: hot rolling to a thickness between 2.0 and 5.0 mm; annealing
the hot rolled strip at 800-1000oC under non.-oxidizing conditions;
cold rolling the strip to a thickness of between 0.5 and 2.0 mm; and
final annealing the cold rolled strip at :' between 1120 and 1200oC for
a period of 2 -5 minutes.
Prior Art:cralaw:red
Stainless steel strips having a 0.2 % yield strength in excess of 50 kg/mm2 are novel and inventive.
Comments: The special technical feature in the product claim is the
0.2% yield strength in excess of 50 kg/mm2. The process steps in claim
2 inherently produce a ferritic stainless steel with a 0.2% yield
strength in excess of 50 kg/mm2. Even if this feature is not mentioned
in claim 2, it is an inevitable result of carrying out the process of
claim 2, and this fact is clearly disclosed in the description.
Therefore said process steps are the special technical feature which
correspond to the limitation in the product claim directed to the same
ferritic stainless steel with the claimed strength characteristics.
Therefore unity is present between claims 1 and 2.
4.2 Claims in the same category
4.2.1 General examples
Example 9
Claims:cralaw:red
1. Electrical plug having a plurality of pins, characterized in that said pins are of hexagonal cross-section of diameter d.
2. Electrical socket having a plurality of elongate bores defining
female contact members, characterized in that said bores are of
hexagonal cross-section of diameter d.
Prior Art:cralaw:red
A prior art document discloses an electrical plug having round pins, and an electrical socket having round bores.
Comments: The special technical feature in claim 1 is the pins of
hexagonal cross-section. Claim 2 involves a corresponding feature to
the special technical feature in claim 1, since the sockets are of
complementary hexagonal form and equal diameter to the pins. Thus, a
technical relationship between the two inventions exists. Consequently
unity of invention is present.
Example 10
Claims:cralaw:red
1. Electrical plug having at least four pins of oval cross-section,
characterized in that said pins are split pins, the joined ends of
which are mounted in the plug body.
2. Electrical socket having at least four socket bores of oval
cross-section defining female contact members, characterized in that
said contact members are held in the socket body by adhesive.
Prior Art:cralaw:red
A prior art document discloses an electrical plug having two pins of
oval cross-section, and a corresponding socket device. A second prior
art document discloses a plug having four pins and a socket with four
socket bores.
Comments: The special technical features of claim 1 are the split pins,
the joined ends of which are mounted within the plug body (i.e. the
only inventive contribution as compared with the prior art as
represented by the teachings of the two prior art documents. None of
the special technical features of claim 1, or features corresponding
thereto, can be found in claim 2, such that there is no technical
relationship between the two inventions. Therefore, unity is lacking
between claims 1 and 2.
Note: In this example, an incorrect approach would consist in using only one prior art
document to determine the special technical feature of claim 1. If, for
example, only the first document was considered, then the following
situation would arise: The special technical features of claim 1 are:
at least four pins, which are split pins, the joined ends of which are
mounted within the plug body. The special technical feature of the four
pins of claim 1 corresponds to the four socket bores of claim 2. This
would lead to the (wrong) conclusion that a technical relationship and,
thus, unity of invention exists between claims 1 and 2.
Example 11
Claims:cralaw:red
1. Transmitter provided with time axis expander for video signals.
2. Receiver provided with time axis compressor for video signals received.
3. Transmission equipment for video signals comprising a transmitter
provided with time axis expander for video signals and a receiver
provided with time axis compressor for video signals received.
Prior art: There is no disclosure or hint in the prior art of the use of time axis expansion/compression in this field.
Comments: The special technical feature in claim 1 is the time axis
expander. The special technical feature in claim 2 is the time axis
compressor, which is a corresponding technical feature to that of claim
1. Claim 3 has both these special technical features. Thus all three
claims are unitary.
Example 12
Claims:cralaw:red
1. Conveyor belt X with feature A.
2. Conveyor belt Y with feature B.
3. Conveyor belt Z with features A + B.
Prior Art:cralaw:red
In the prior art conveyor belts X, Y and Z without features A or B are disclosed.
Comments: Feature A in claim 1 is the special technical feature which
defines the contribution over the prior art. This feature is not
related with the feature B in claim 2. Unity of invention exists
between claims 1 and 3, but not between claims 1 and 2. Also there is
unity between claims 2 and 3.
Example 13
Claims:cralaw:red
1. Control circuit for a d.c. motor, said circuit having feature A.
2. Control circuit for a d.c. motor, said circuit having feature B.
3. A vehicle including a d.c. motor with a control circuit having feature A.
4. A vehicle including a d.c. motor with a control circuit having feature B.
Prior Art:cralaw:red
Control circuits for d.c. motors are well-known, but features A and B
are individually new and inventive. However, features A and B are
completely unrelated.
Comments: The special technical feature in claim I is feature A, which
is present in claim 3, such that unity exists with claim 3, but not
with claims 2 and 4. The special technical feature in claim 2 is
feature B. which is present in claim 4, such that unity exists with
claim 4, but not with claims 1 and 3.
Example 14
Claims:cralaw:red
1. A display comprising a two-dimensional array of groups (A) of white
light sources, each group (A) of sources being associated with a
respective set (B) of differently coloured filters.
2. A display according to claim I in which the filters in each set (B)
are red, blue and green of particular transmission wavelengths (C).
3. A display according to claim 1 in which the light sources are filament lamps (0).
Prior Art:cralaw:red
(i) Situation 1: A prior art document discloses a display comprising a two-dimensional
array of white light sources. Claim 1 is novel and inventive in the light of said prior art
document.
(ii) Situation 2: A prior art document discloses a display comprising a
two-dimensional array of white light sources. A second prior art
document discloses a linear array of groups of white light sources,
each group of sources being associated with a respective set of
differently coloured filters.
Claim 1 is not inventive in the light of said two prior teachings.
Comments:cralaw:red
(i) Situation 1: Claims 2 and 3 are properly drafted dependent claims
defining detail features of the subject-matter of claim 1. Therefore
there is automatically unity of invention between claims 1, 2 and 3.
(ii) Situation 2: Claim 1 must be deleted for lack of inventive step.
The remaining claims 2 and 3 must now be treated as independent claims
to determine whether or not there is unity of invention. The special
technical feature (c) in claim 2 is that the filters in each group of
light sources are red, blue and green of particular transmission
wavelengths. This special technical feature, or features corresponding
thereto, is not to be found in claim 3.
Therefore, unity is lacking a posterior between claims 2 and 3.
Example 15
Claims:cralaw:red
1. A filament for an electric lamp, said filament having a special construction A.
2. An electric lamp B comprising a filament having a special construction A.
3. A searchlight comprising a lamp B having a filament having a special
construction A, said lamp being mounted by a swivel arrangement C.
Prior Art:cralaw:red
A prior art document discloses a filament for a lamp, there being no disclosure or hint of the special construction A.
Comments: The special technical feature in claim 1 is the special
construction A for the filament, which is present in all three claims,
thereby providing a special technical feature needed to fulfil the
requirements of unity of invention. chanroblesvirtualawlibrary
Example 16
Claims:cralaw:red
1. A marking device for marking animals, comprising a disc-shaped
element with a stem extending normally therefrom, the tip of which is
designed to be driven through a body portion of the animal to be
marked, and a securing disc element to be fastened to the protruding
tip of the stem on the other side of the body portion.
2. An apparatus for applying the marking device of claim 1, constructed
as a pneumatically actuated gun for driving the stem of the disc-shaped
element through the skin, and provided with a supporting surface
adapted for taking up a securing disc element, to be placed at the
other side of the body portion in question of the animal to be marked.
Prior Art:cralaw:red
A prior art document discloses a marking device for animals. However,
no disc-shaped element with a stem and a securing disc element is
disclosed or hinted at in the prior art.
Comments: The special technical feature in claim 1 which makes the
contribution over the prior art is the disc-shaped element with a stem
and a securing disc element to be fastened to the tip of the stem. The
corresponding special technical feature in claim 2 is the pneumatically
actuated gun for driving the marking device through the body part, and
the supporting surface for the securing disc element. Hence unity
exists between claims 1 and 2.
Example 17
Claims:cralaw:red
1. Compound A.
2. An insecticide composition comprising compound A and a carrier.
Prior Art
In the light of the prior art compound A is novel and inventive.
Comments: Unity exists between claims 1 and 2. The special technical feature common to both claims is compound A.
Example 18
Claims:cralaw:red
1. An insecticide composition comprising a compound of the general formula A and a
carrier. [Formula A comprises various Compounds a1, a2, ]
2. Compound a1.
Prior Art:cralaw:red
Certain specific compounds covered by the general formula A are known, but not as
insecticides. The compound a1 is novel and inventive.
Comments: All compounds of general formula A are not claimed in claim 2 as, for
example, some of them lack novelty. Nevertheless there is still unity
between the subject-matter of claims 1 and 2, provided a1 possesses
insecticidal activity which is the special technical feature for the
compounds of general formula A in claim 1.
Example 19
Claims:cralaw:red
1. Protein X.
2. DNA sequence encoding protein X.
Prior Art:cralaw:red
In the light of the prior art protein X is novel and inventive.
Comments: Expression of the DNA sequence in a host results in the
production of a protein which is determined by the DNA sequence. The
protein and the DNA sequence exhibit corresponding special technical
features. Unity exists between claims 1 and 2.
4.2.2 Examples concerning intermediate and final products
Example 20
Claims:cralaw:red
1. Amorphous polymer A.
2. Crystalline polymer A.
Prior Art:cralaw:red
The polymer A is novel.
Comments: In this example a film of the amorphous polymer A is
stretched to make it crystalline. Here unity exists because there is an
intermediate/final product relation in that amorphous polymer A is used
as a starting product to prepare crystalline polymer A. For purposes of
further illustration, assume that the polymer A in this example is a
specific polyisoprene. Here the intermediate, amorphous polyisoprene,
and the final product, crystalline polyisoprene, have the same chemical
structure.
Example 21
Claims:cralaw:red
1. Novel compound having structure A. [Intermediate]
2. Product prepared by reacting A with a substance X.
[Final product with unknown structure]
Prior Art:cralaw:red
No relevant prior art document was found in the search.
Comments: In this example the structure of the product of claim 2 (the
final product) is not known. Unity exists if there is evidence which
would lead one to conclude that the , characteristic of the final
product which is the inventive feature in this case is due to the
intermediate. For example, the purpose of using the intermediate is to
modify certain properties of the final product. The evidence may be in
the form of test data in the specification showing the effect of the
intermediate on the final product. If no such .evidence exists then
there is no unity on the basis of an intermediate-final product
relationship.
Example 22
Claims:cralaw:red
1. Reaction product of A and B. (Intermediate with unknown structure).
2. Product prepared by reacting the reaction product of A and B with
substances X and Y. (Final product with unknown structure).
Prior art:cralaw:red
No relevant prior art document was found in the search.
Comments: In this example the structures of the products of claim 1
(the intermediate) and claim 2 (the final product) are unknown. Unity
exists if there is evidence which would lead one to conclude that the
characteristic of the final product which is the special technical
feature in the case is due to the intermediate. The evidence may be in
the form of test data in the specification showing the effect of the
intermediate on the final product. If no such evidence exists then
there is no unity on the basis of an intermediate-final product
relationship. chanroblesvirtualawlibrary
4.3 Alternatives in a single claim I Markush practice
Example 23 -Common Structure Claim
1. Pharmaceutical compounds for enhancing the capacity of the blood to absorb oxygen, of the formula

wherein R1 is selected from the ~roup consisting of phenol, pyridyl,
thiazolyl, triazinyl, alkylthio, alkoxy and methyl; R2 -R are methyl,
benzyl or phenyl.
Prior Art:cralaw:red
For the purpose of this example it is assumed that the search reveals
that no indolyl derivatives are known which possess CI pharmaceutical
activity and, in particular, the property of enhancing the capacity of
the blood to absorb oxygen. The claimed compounds are novel and
Inventive.
Comments: Claim 1 defines various alternatives of individual chemical
compounds. The indolyl moiety is the significant structural element
which is shared by all of these alternatives. Since all the claimed
compounds are alleged to exhibit the same pharmaceutical activity (i.e.
enhancing the capacity of the blood to absorb oxygen), the indolyl
moiety is the special technical feature. This special technical feature
is common to all alternatives covered by claim 1. Thus, claim 1 is
unitary.
5 Chemical Problems
The following text is designed to help substantive examiners in dealing
with problems that arise when considering chemical applications. The
examples cover different types of subjects such as patentability,
medical methods, novelty, inventive step, etc., their common theme
being that they are in the chemical area.
The following definitions apply:cralaw:red
Compound -a single chemical substance.
Composition -a mixture of compounds (N.B. usually containing one or
more active compounds, which do not react chemically with one another).
Product -a compound, a composition or a reaction mixture (N.B. such
products may be obtained by chemical or physical process or by
extraction).
Index of examples
5.1 Patentable Inventions
1. Compounds and compositions having a special scent or flavour
2. Unstable compounds
3. "Intermediate products"
5.2 Inventions relating to surgery. therapy or diagnostic methods
4. Allowable claims
5. Known physiological effect
6. Known pharmaceutical use
7. Second medical indication
8. Allowable "second medical indication" claims
9. Synergistic effects (kit of parts)
10. Prevention or treatment of parasitic infestations
5.3 Novelty
11. Limitation of a claim concerning a family of chemical compounds lacking novelty
12. Novelty in connection with composition ranges
13. Novelty of chemical compounds
14. Novelty of stereoisomers
15. Novelty destroying effect of non-repeatable prior art
16. Novelty of naturally-occurring products
5.4 Inventive step
17. Inventive step in chemical compounds
18. Comparison of properties. The need to call for comparative tests to establish an Inventive step
19. Inventive selection
20. Inventive step in claims for "intermediate chemical products"
21. Inventive step of a product based on the process for its production
5.5 Disclosure of invention
22. Chemical compounds -statements of use or effect
23. Amendment of the disclosure after the filing date
24. Claims to compounds which cannot be prepared
5.6 The claims
25. Claims containing functional features
26. Definition in claims by parameters
27. Analogy processes
28. Independent claims of the same scope
29. Claims containing general expressions
5.7 Microbiological Processes. genetic manipulation and Products obtained by such Procedures.
30. Novelty of naturally-occurring polymers
31. Novelty-destroying effect of micro-organisms mentioned in the prior art
32. Patentability of claimed DNA sequences
33. Claims to recombinant vectors
34. Hybridomas and monoclonal antibodies
35. Claims to biological material per se
36. Microbiological inventions
37. Generalization in the field of microbiological inventions
38. Terminology in claims for biological material
39. Novelty of biological material
40. Inventive step for biological material
41. Biological material -availability to the public
42. Characterization of deposited biological material
43. Priority provisions for microbial inventions.
5.1 Patentable inventions
Example 1 Compounds and compositions having a special scent or flavor
(1) Should new chemical compounds or compositions, which exhibit a
special scent or flavor (making them useful, for instance, in the
perfumery or food industry) be refused as being aesthetic creations?
Answer: No. Such a compound or composition should be judged like any
other chemical compound or composition whose properties are not merely
aesthetic.
(2) Should new compositions possessing only the aggregate known scent
or flavour of its ingredients, or processes for producing such a
composition by mere admixture, be refused as lacking inventive step?
Answer: Yes. However, should the composition exhibit an unexpected
effect such as an intensification of scent or flavor, additional
stability, or prolonged persistence of scent or flavor, then the
composition should be regarded as inventive.
Example 2 Unstable Compounds chanroblesvirtualawlibrary
Can an unstable compound be protected by a patent? chanroblesvirtualawlibrary
Answer: Yes, if its instability does not prevent it being industrially
applied i.e. made or used in any kind of industry, including
agriculture. The unstable compound must be defined unambiguously in the
application (i.e. separated* and identified). The fact that its life
may be very short is no bar to patentability so long as the above
requirements are satisfied.
*If the unstable compound cannot be separated, then it cannot be
protected by a patent but e.g. a solution containing it could be
patentable. chanroblesvirtualawlibrary
Example 3 Intermediate Products chanroblesvirtualawlibrary
Sometimes reference is made to chemical "intermediate products ". For
the purposes of this example, chemical intermediate products are
defined as compounds which do not necessarily have any useful direct
application but which serve as starting substances for producing other
chemical substances ("end products") by a chemical reaction. In some
cases, intermediate products are formed in the course of a claimed
process. These intermediates may sometimes be separated but are
sometimes so short-lived that they cannot be separated (they may be
identified spectroscopically, for instance) or are only hypothetical.
For the patentability of chemical intermediates, the normal criteria of
novelty, industrial applicability and inventive step have to be
satisfied.
Are new chemical intermediates patentable in the following cases if : chanroblesvirtualawlibrary
(1) the chemical intermediate can be separated? chanroblesvirtualawlibrary
(2) The chemical intermediate exists as ions, which cannot be
separated, or as hypothetical stages in a reaction mechanism? chanroblesvirtualawlibrary
Answers: chanroblesvirtualawlibrary
(1) Yes:cralaw:red
Examples of this type are diazonium salts Ar-N+=NX- which are not
isolated from the reaction mixture obtained on their preparation. The
reaction mixture as such is used for further chemical reactions for the
preparation of an end product. e.g. azo-dyes. chanroblesvirtualawlibrary
ArNH2
+ NaNO2
+ 2HCI --- > (ArN
N)+CI
-
+ NaCI + 2H20
I
intermediate
diazonium-salt solution
I
I
ArN=NR
+ HCI
(2)
No:
Examples
of this type could be carbenium ions which form intermediate stages in a
reaction
and are incapable of isolation.
(CH3)3C-CH=CH2
+ HCI ---->
[(CH3)3C-CH-CH+
-CH3]
+ CI
-
/
\
/ carbenium ion I \
/
\
(CH3)3C-CHCI-CH3
[
(CH3)2C+
-CH(CH3)2]
CI
-
I
3,3-dimethyl-2-chlorobutane
rearrangement to carbenium ion II
I
I
2.
3-dimethyl-2-chlorobutane
5.2 Inventions relating to surgery. therapy. or diagnostic methods
Example 4 Allowable Claims chanroblesvirtualawlibrary
In the case where:cralaw:red
(1) the compound X is new, chanroblesvirtualawlibrary
(2)
the compound X is not new and the state of the art does not comprise
its use in surgery, therapy, or diagnostic methods, chanroblesvirtualawlibrary
(3) the compound X is not new and the state of the art comprises its
use in surgery, therapy, or diagnostic methods, are the following
forms of claim allowable? chanroblesvirtualawlibrary
(a) compound X for use as a medicament chanroblesvirtualawlibrary
(b) compound X for use as an analgesic chanroblesvirtualawlibrary
(c) compound X for use in curing disease Y chanroblesvirtualawlibrary
(d) composition A containing X for use in curing disease Y (composition A may be generally defined) chanroblesvirtualawlibrary
(e) medicament containing the compound X chanroblesvirtualawlibrary
(f) use of X in a composition A for the curing of disease Y chanroblesvirtualawlibrary
(g) use of X as a medicament for the curif1g of disease Y chanroblesvirtualawlibrary
(h) use of X for the curing of disease Y chanroblesvirtualawlibrary
(i) use of X for preparing a medicament chanroblesvirtualawlibrary
(j) use of X for the manufacture of a medicament for curing disease Y chanroblesvirtualawlibrary
Answers: A summary of the following explanation is to be found at the end of the example. chanroblesvirtualawlibrary
1) X is new: chanroblesvirtualawlibrary
(a),(e),(i),(j) Yes chanroblesvirtualawlibrary
(b),(c),(d) Yes, more than one such claim is allowable in the same application chanroblesvirtualawlibrary
(f) No, since the scope of the cl1im is not clear and this type of
claim could be interpreted as a method claim which is excluded from
patent protection. chanroblesvirtualawlibrary
(g),(h) No, claims of the above form are method claims which are excluded from patent protection. chanroblesvirtualawlibrary
2) X is known, but has not been described a having pharmaceutical properties: chanroblesvirtualawlibrary
(a),(b),(c),(d),(e): Yes, providing a specific first medical use is
disclosed, then a general claim such as (a) or (e), to the use of
compound X as a medicament is allowable. Where there are claims to
specific first medical uses as exemplified by (b),(c) and (d), then
more than one such claim is allowable in one application.
(f),(g),(h), No, as under 1) above
chanroblesvirtualawlibrary
(j)
Yes. chanroblesvirtualawlibrary
3) X is known and has been described as having pharmaceutical properties: chanroblesvirtualawlibrary
(a),(b),(c),(e) No, in view of lack of novelty chanroblesvirtualawlibrary
(d) No, if the composition A itself was known as a medicament
Yes, if the composition A itself was not known, or not known as a medicament.
(f),(g),(h) No, as under 1) above.
(i) No, as a medicament has also been prepared using X
(j) Yes.
N.B.: In the case of a first medical indication, the instructions (a)
to (j) apply to claims with equivalent wording. The examiner must
however ensure that the wording of the claim under consideration has
the same sense as examples (a) to (j) given above. In the case of a
second medical indication the examiner is advised to allow only the
wording set out in a). chanroblesvirtualawlibrary
Example 5 Known physiological effect chanroblesvirtualawlibrary
Does the prior disclosure of a physiological effect of a product
constitute use in an area excluded from patent protection render a
claim to a product for use in such method non-" patentable for lack of
novelty?
Answer: It depends upon the manner in which the physiological effect
has been disclosed. If the disclosure implies that the product has been
previously medicinally administered or the physiological effect is
reported in an investigation aiming at development of medicines, the
answer is yes. The existence of a physiological effect does not
necessarily imply that an excluded method has been previously effected.
For example, a compound may affect the biological working of the body
yet no medical or therapeutic effect may have been foreSeen.
Example 6 Known pharmaceutical use chanroblesvirtualawlibrary
(a) Is a general statement of pharmaceutical application for a
known product in the prior art considered to constitute an excluded
method? chanroblesvirtualawlibrary
Answer: No unless it is obvious from the type or structure of the
product previously disclosed that a certain pharmaceutical use was
intended (for instance in the case of penicillins). chanroblesvirtualawlibrary
(b) Will a specified pharmaceutical application for a known product having no
experimental support or other information in a prior art document be considered to constitute an excluded method. chanroblesvirtualawlibrary
Answer: Yes, except if the examiner is convinced that the statement in
the prior art document is incorrect, e.g. in view of evidence from the
applicant that this statement is incorrect.
Example 7 Second medical indication chanroblesvirtualawlibrary
When a second medical property has been discovered for a known active
compound X can a product claim directed to a composition containing the
compound X; be allowed? chanroblesvirtualawlibrary
Answer: Yes, provided the requirements of novelty and inventive step are met. chanroblesvirtualawlibrary
Novelty -when the composition is defined broadly as X + diluent (not
specified), then this composition would generally not be novel.
Limitation of the claim to X + a specified diluent (or other specified
additive), possibly in specific proportions, may be necessary for
establishing novelty.
Inventive step -could be accorded on the basis of the medical effect or
other advantages, for example either an unexpected medical effect due
to the compound itself, or a synergistic action from the combination of
X with other ingredients.
Example 8 Allowable second medical indication claims chanroblesvirtualawlibrary
Is the following claim acceptable? chanroblesvirtualawlibrary
"Compound X for use in curing carcinoma". chanroblesvirtualawlibrary
Where compound X is known as a component in a medicinal composition
only in combination with 1 or more active compounds for use in curing
anorexia and where compound X is not disclosed as having, in itself, a
therapeutic effect in said composition? chanroblesvirtualawlibrary
Answer. YES, from the point of view of novelty. chanroblesvirtualawlibrary
The application constitutes the first recognition that the compound X
by itself has medicinal properties and thus the first use of compound X
for these medicinal properties. chanroblesvirtualawlibrary
Example 9 Synergistic effects (kit of parts) chanroblesvirtualawlibrary
In the state of the art compounds X and Yare known and the use of each
of them in therapy has been described. The administration of X and Y in
association is now found to lead an unexpected, new synergistic effect
in the treatment of carcinoma. In these circumstances is the following
claim allowable?
"Pharmaceutical products for use in carcinoma, consisting of an
association of compound X and compound Y, in which the two active
compounds are adjacent but separate." chanroblesvirtualawlibrary
Answer: Yes. Patentability is acknowledged for a "kit-of-parts" in which the active
compounds are physically separated, provided that the use of both
compounds, either simultaneously, separately or sequentially, gives a
new and unexpected therapeutical effect. chanroblesvirtualawlibrary
Example 10 Prevention and treatment of parasitic infections chanroblesvirtualawlibrary
Is a method of treatment of parasites found on or within the human or animal body excluded from patentability? chanroblesvirtualawlibrary
Answer: (i) Yes, if it is concerned with a human or animal internal
parasite, or with the prevention or treatment of infestation by an
external parasite, when this is considered as a treatment of an illness
of which it is the vector or cause.
(ii) No, in all other cases. chanroblesvirtualawlibrary
Commentary: The method of treatment is excluded from patentability when
it is concerned with a surgical or medical treatment of the human or
animal body. The response to this question will vary according to
whether the prevention or treatment is therapy (or surgery) or not. The
notion of therapeutic treatment is connected with the notion of a human
or animal illness. However, the presence in itself of certain external
parasites on the human or animal body should not always be considered
as an illness. chanroblesvirtualawlibrary
Examples: 1. The treatment of the human body to remove tape worms will be a therapeutic treatment.
2. The treatment or prevention of itch mites in the case of the
treatment of scabies which they provoke will be a therapeutic
treatment. chanroblesvirtualawlibrary
3. An external treatment of fleas in the absence of an illness will not be a therapeutic treatment.
5.3 Novelty
Example 11 Limitation of a claim concerning a family of chemical compounds lacking novelty
A patent application claims chemical compounds of general formula:cralaw:red
'N'--- X,
wherein 'N' is an organic nucleus and X may be an alkyl group. In the
original application three particular compounds are mentioned wherein:cralaw:red
X = methyl (C1) X = propyl (C3) and isopropyl (C3)
In the case in which a prior publication discloses decyl(C10) what limitation of the claim may be accepted?
Answer: In order not to be unallowable as new subject-matter, the
limitation must be explicitly or implicitly derivable from the content
of the original application or must be in the form of a disclaimer.
(a) Content of the original application.
It is Seen that, in the original application, are disclosed in addition to the general formula
'N'---- X
-the particular compounds C1 and C3 (explicit)
-the group of compounds C1 to C3 (implicit)
(b) Novelty.
In this case it is considered that the general formula is no longer novel, but what remain novel are
-the compounds C1 and C3
-and the general formula 'N'--- X in which X may be an alkyl group other than C10'
From the point of view of novelty and the original disclosure, one is
led to accept, formulated in any way, the following limitation:cralaw:red
(a) -C1
(b) –C3
(c) –C1 and C3
(d) -C1 to C3
(e) -X = Cn (n =/= 10).
The limitations (a) to (d) are narrow and sufficiently removed from the
example of the prior document (C10). The examiner must ensure, however,
that the compounds are not selected arbitrarily but with a particular
purpose,
The limitations (c) to (e) should only be accepted if the examiner is
sure that he is dealing with a unitary group of compounds.
N.B.1: "X = C1 to C9 and C11 and above" should not be accepted since
C9 and C11 are not part of the original disclosure. Accept only the
disclaiming definition X = Cn (n = 10 excepted). chanroblesvirtualawlibrary
Inyentive Step.
If the prior publication describing the C10 compound was published
before the priority date of the claim, then it is necessary to
establish whether the limited claim is inventive. chanroblesvirtualawlibrary
N.B.2: When during examination, a very broad main claim has been
restricted to render the claim novel (e.g. by the incorporation of
matter which appeared from the original application to be of only minor
importance) the substantive examiner should consider whether or not a
further search is required.
Example 12 Novelty in connection with composition ranges
Question 1 :cralaw:red
When compositions are defined by a range, what criteria are to be employed in determining novelty?
Answer:cralaw:red
1. Exemplified specific values are novelty-destroying if they lie within a claimed range.
2. The end-values of a published composition range are
novelty-destroying if they automatically lead to a specific
composition.
This is generally the case with binary compositions. For example, the
publication of the composition range 40 to 70% A, 60 to 30% B is
novelty-destroying for the following specific compositions:cralaw:red
(1) 40% A, 60% B, and (2) 70% A, 30% B
An application that claims for example, the overlapping range 65 to 85%
A; 35 to 15% B has to include a disclaimer to the composition 70% A:
30% B in order to be novel.
In composition ranges for compositions having more than two components
the end-values do not normally lead to a specific composition (assuming
that no further information is given in the publication). The
composition ranges: A: 10 -30%, B: 25 -40%, C: 40 -60% do not lead
directly to any specific composition.
In contrast to the above, the composition ranges: A': 10 -30% B': 25
-40% C': 30 -65% have clear specific limiting compositions, namely (1)
30% A', 40% B', 30% C' and (2) 10% A', 25% B', 65% C'- which are to be
regarded as explicit disclosures.
The general rule is: If the limiting value of a component in a
published composition range leads automatically to distinct values for
the other components (possibly by means of information in the
publication), then the respective composition is to be regarded as
explicitly disclosed.
3. Where a prior publication discloses a broader or overlapping range the following principles are to be applied:cralaw:red
N.B.: Inventiveness: For those areas which are common to both
application and the citation the known requirements for inventiveness
of selections are, of course, to be employed.
Question 2 =
When a disclaimer is employed to establish novelty with respect to an
overlapping range disclosed in a prior art document, what format should
the disclaimer have and which disclosure may be used as a basis for the
wording of the disclaimer?
e.g. Range claimed in application x = 600 to 10,000.
Range disclosed in prior art x = 240 to 1,500
Answer: The disclaimer should form part of the claims and not only the
description. The wording of the disclaimer should be based either upon
the original disclosure (e.g. a value originally disclosed in the
description or an example) or on the content of the prior art document.
In the above case the range, x = above 1,500 to 10,000 is acceptable
whereas x = 1,500 to 10,000 is not as the value 1,500 was not disclosed
in the original application.
Example 13 Novelty of chemical compounds
Question 1
What criteria are to be used in determining whether or not a chemical
compound, a composition, an alloy etc. is novel with respect to a prior
document?
Answer
i) Normally a chemical product (compound, a composition, an alloy
etc.), is considered to be known if the product has been mentioned in a
prior art document and the information in and the teaching of the prior
art document together, where appropriate, with common general knowledge
at the effective date of the document, would enable the man skilled in
the art to make and separate the product, or in the case of a product
of nature, only to separate the product. The term "mentioned" is to be
understood as referring to a precise definition of the product either
by (a) its name, (b) its formula, (c) its parameters or (d) as the
product of a process.
Thus in cases (a) and (b) the mere name or chemical formula will be
novelty-destroying to a later application, provided that the name or
chemical formula is sufficient to define the product with a degree of
precision equal to or greater than that used in the later application.
In case (d) if the prior art document discloses starting materials in
combination with the process in such a manner that their use in the
process inevitably results in the claimed product, then the prior art
document is novelty-destroying.
ii) If, in response to an objection of lack of novelty, the applicant
produces evidence proving that it would not have been possible for a
man skilled in the art, to prepare and to separate the compounds under
the conditions set out in the cited document, the cited document is not
novelty-destroying.
iii) It should also be noted that a general formula does not destroy
the novelty of a single member or a sub-group within the large group
corresponding to the general formula.
Examples: "Halogen" does not destroy the novelty of chlorine. "Lower alkyl" does not destroy the novelty of C2H5.
On the other hand, a list of mentioned chemical compounds destroys the
novelty of every member of the list, provided it can be prepared and
separated, as stated in the first paragraph.
Example: A list containing the members:cralaw:red
Ø-CH3
,Ø-C2H5 ,Ø-i-C3H7 ,Ø-n-C3H7, Ø-n-C4H9
destroys the novelty of: Ø-C2H5.
However, a reference to C1-C4 compounds destroys the novelty of the C1
and C4 compounds, i.e. the explicit ends of the range. On the other
hand, if the C4 compound has several isomeric forms, the novelty of
each individual isomer is not necessarily destroyed by a reference to
the C1-C4 compounds. In this case the name or the formula may be
insufficient to precisely define the product. The examiner must
determine whether the product of the prior art document is identical
with the product of the application, for example by comparison of the
physical, chemical or other properties of the two products under
consideration.
Question 2
Are physico-chemical data, medical data or other verifying data for
chemical compounds in a publication or in a prior patent application,
required for an opinion that the compound in question is known?
Answer: No,
but they might be used to refute an argument of lack of novelty.
Example 14 Novelty of stereo isomers
A racemic mixture is an optically inactive mixture of equal parts of a
dextro- and a laevo-compound. If a racemic mixture is known will the
two optically active constituents also be considered to be known,
(a) if the constituent has not previously been mentioned express is verbis?
(b) if the constituent has been mentioned previously?
(c) if it is merely said in a prior art document that racemic mixtures may be resolved into their constituents?
Answers:cralaw:red
(a) No, but exceptionally, if in a prior art document, a
particular stereoisomer is not explicitly mentioned but there is
adequately described a process for reacting a starting compound such
that, upon carrying out the process on the starting compound, the
particular stereoisomer would inevitably be produced, then the prior
art is novelty-destroying.
(b) Yes, provided the information in, and teaching of the prior art
document, as well as general knowledge at the effective date of the
document, would enable the skilled man in the art to carry out the
separation and isolation. It should be noted, that the separation and
isolation have not necessarily to have been carried out to destroy
novelty.
(c) No.
N.B. The inventive step of the optically active constituent has also
to be considered. This could be established by comparing an effect
(e.g. therapeutic) of the stereoisomer with the state of the art.
Example 15 Novelty destroying effect of non-repeatable prior art
In an earlier publication a specified chemical compound is described by
a formula and a characteristic property. e.g. its melting point.
Possibly also the method for its manufacture is given in the same
article or alternatively in some other publication.
An application is later filed claiming the same compound. The applicant
states that it was not possible to make the compound using any of the
known methods. He himself has invented a new method by which he
succeeded in manufacturing the compound.
Should the compound be considered to be novel in spite of the fact that
it has been apparently been prepared earlier in some way and also
characterized, although its preparation later proved to be impossible
applying the known methods?
Answer: A chemical product is considered to be known if the product has
been mentioned in a prior art document and the information in and the
teaching of the prior art document together, where appropriate, with
the general knowledge at the effective date of the document, would
enable the skilled man to make and separate the product.
The examiner should ask the applicant for convincing evidence that
preparation of the compound according to the earlier publication is
impossible. If then, the examiner is convinced that no method was
available at the date of the earlier publication for the manufacture of
the specific chemical compound, the compound is novel in spite of the
fact that the characteristics given for the prior art compound are
correct.
The novelty of the compound could also be based on the fact that this
compound, although designated by the same name, exhibits different
characteristics from the previously designated compound.
Example 16 Novelty of naturally occurring products
A naturally-occurring product referred to as a hereafter as Product A
is claimed in an application. Is the product novel in the following
circumstances?
Case 1. The prior art discloses a product having the same activity but
does not indicate its formula nor any physico-chemical characteristic
or parameter, or its way of manufacture that enables the examiner to
establish whether the prior art product is the same as or differs from
Product A.
Answer. Since the activity alone is insufficient to unambiguously define the product of the prior art, Product A is new.
Case 2. Product A is claimed as having a specific activity and is
described in terms of physico-chemical characteristics. The prior art
discloses a product having the same activity and certain other
physico-chemical characteristics.
Answer. To establish whether Product A and the prior art product are
the same the examiner can only employ characteristics which
unambiguously identify a product. A tentative objection of lacking
novelty must be raised in specific circumstances. The examiner must
ensure that Product A which is identified by certain parameters is not
a mere re-writing of a product that is already known and which is
identified by other parameters.
Case 3. The prior art discloses a product in the form of a mixture with
an impurity B in an amount of up to X%. The application claims a purer
form in which B is in a smaller amount x%.
Answer. The criteria for selection inventions are to be employed in
determining whether or not the purer product is novel. The prior art is
considered to relate to a composition of the Product A and the impurity
whereas the purer product is a selection within this composition
containing a smaller amount of impurity. The degree of impurity of the
claimed product must be substantially below that encountered in the
prior art and the claimed purer product must achieve a specific purpose
or specific technical effect which differs from that of the prior art
(purposive selection). If these criteria are fulfilled then the purer
claimed product is novel.
Case 4. Product A is known in a form which has been purified but the
formula was not disclosed. The applicant now says that he has been able
to identify the formula of Product A and claims it.
Answer. A claim directed to the formula of a known product lacks novelty.
5.4 Inventive step
Example 17 Inventive step in chemical compounds
What is required to accord inventive step to a novel chemical compound?
a) if the compound is not structurally close to known compounds?
Answer: In these rare cases the examiner should decide that there is
inventive step in the structure and no use or effect has to be
considered.
b) if the compound is structurally close to known compounds?
Answer: To accord an inventive step the novel compound must exhibit a
use or an effect which is unexpected. Fulfilment of this condition is
sufficient for inventive step. This un-expected use or effect may be
entirely different from the known uses or known effects of the known
compounds. Alternatively, it may be a substantial improvement in an
effect of the same kind as is exhibited by the known compounds. Further
a use or effect is to be considered as unexpected when no use or effect
at all has been explicitly described in the prior art or is derivable
from general knowledge.
The indication of an unexpected use or effect which supports the
patentability of a new compound may be filed later than the original
filing date of the application. However, this is new subject-matter so
this effect generally cannot be introduced into the description or the
claims. chanroblesvirtualawlibrary
Note 1: To support an objection of lack of inventive step it is
sufficient that the skilled person would have regarded the applicant's
novel compound as something he could make or use. It is not necessary
that the citation incites the skilled person to make or use the novel
compound. Nevertheless it is necessary, in order to support the
objection, to give some reasons why these properties are expected and
not simply to write that the novel compound is structurally close to
the known compounds. chanroblesvirtualawlibrary
Note 2: The fact, that the documents cited in the search report are
not sufficient by themselves to raise such an objection does not
prevent the examiner from C raising it if he thinks that these
documents combined with general knowledge in the field concerned will
allow him to object. This may happen, for example, when the objection
of lack of inventive step is based on only one document, combined with
general knowledge not contained in a document cited in the search
report. In such a case the examiner may, if necessary, do a quick
search in reference books in order to prove his assertion concerning
common general knowledge. He should nevertheless object, whatever the
result of this search may be. If the applicant contests the objection
there are three possibilities: chanroblesvirtualawlibrary
- the examiner waives the objection because he is convinced by the applicant's arguments
- the examiner maintains the objection because he is satisfied that
even without a document the objection is soundly based chanroblesvirtualawlibrary
- the examiner does an additional search and acts according to the result of this search. chanroblesvirtualawlibrary
Note 3: In some cases inventive step could be based upon the
inventiveness f the process by which the novel compound is obtained. chanroblesvirtualawlibrary
Note 4: In order to establish inventive step of a claim which relates
(i) to a known compound for a specific pharmaceutical use in surgery
or treatment or (ii) to compositions containing known active substances
for a specific pharmaceutical use in surgery or treatment, the examiner
will generally have to ensure that sufficient details of
pharmacological effects are available. It may be necessary for the
examiner to request details of qualitative effects or quantitative
data, exceptionally in the form of comparative tests, from the
applicant.
Example 18 Comparison of properties. The need for comparative tests chanroblesvirtualawlibrary
Is it necessary, in order to establish an inventive step, to make a
comparison between a specific effect (e.g. analgesic) of the new
compound (e.g. Z-C2Hs) and the effect of a structurally-related
compound (e.g. Z-CH3) in the case of:cralaw:red
(a) no effect being mentioned in the prior art document, or chanroblesvirtualawlibrary
(b) a different effect (e.g. as plasticiser) being mentioned in the prior art document, or chanroblesvirtualawlibrary
(c) the same effect being mentioned in the prior art document, or ,. chanroblesvirtualawlibrary
(d) a related effect (e.g. anaesthetic) being mentioned in the prior art document? chanroblesvirtualawlibrary
Answer:cralaw:red
(a) No.
(b) No.
(c) Yes, when the applicant does not provide any evidence
that would render the existence of an inventive step plausible, a
comparison is necessary to demonstrate any new and surprising technical
advantage that may be present, particularly in the case of a claimed
range of values where one of the limits of the range is close to a
value disclosed in a prior art document.
(d) Only if it is obvious that a man skilled in the art could expect
that the known compound would also have an analgesic effect.
chanroblesvirtualawlibrary
Note: Applicants are reluctant to make comparative tests on the grounds
of the expense and time involved and they should be called for only
when absolutely necessary. The examiner should rather ask for any kind
of proof of the presence of a new and surprising technical advantage,
than explicitly for comparative tests. Comparative tests may be needed
when the compound falls within the area of a known general formula or
is just outside such a formula or is structurally closely related to a
known compound. Whenever a comparative test is made, the comparison
must be with the technically closest prior art, in a comparable type of
use. For a claimed organic compound, the compound which must be used
for the comparative test, is that prior art compound with the closest
possible similarity from the point of view of both structure and
effect. In principle the compound used for the comparative test should
be a known compound. In exceptional cases a fictive compound may be
used. chanroblesvirtualawlibrary
Example 19 Inventive selection chanroblesvirtualawlibrary
Suppose that a prior art document discloses a group of chemical
compounds expressed by the general formula A-R, in which A is the major
part of a molecule and R is an alkyl group, having 1-20 carbon atoms.
An applicant now claims a narrow group of these compounds, with 3-5
carbon atoms in the alkyl group whereby the new narrow group lies
within the earlier group.
Suppose that no chemical compounds within this narrow group are
mentioned explicitly in the prior art document, i.e. either by name or
by formula, nor by physico-chemical data, the narrow group itself has
not been mentioned explicitly, and the examples in the prior art
document are remote from the narrow group (e.g. C16,C17) Furthermore,
the compounds of the narrow group all exhibit a technical effect or
property which is not disclosed in the prior art document. chanroblesvirtualawlibrary
Will the compounds of the narrow group according to the later
application be judged to show inventive selection and represent a
patentable invention? chanroblesvirtualawlibrary
Answer:cralaw:red
Novelty: From what is said above, and having regard to the conditions
enumerated in example 23 above, the small group of compounds is novel. chanroblesvirtualawlibrary
Inventive Step : If the above-mentioned technical effect or property
is' unexpected, an inventive step is present. The invention would
therefore be patentable. chanroblesvirtualawlibrary
Processes: Similarly a process can be considered inventive where the
selection of narrow group of starting compounds from a larger group of
known starting compounds leads to an unexpected effect, such as an
unexpected increase in yield when compounds of the narrow group are
reacted.
Example 20 Inventive step in claims for intermediate chemical products chanroblesvirtualawlibrary
Preliminary remark: The intermediate chemical products considered here
are those for which no direct activity can be used to establish
inventive step. They are used to prepare inventive "subsequent
products" or are intermediates in an inventive process. chanroblesvirtualawlibrary
Question: Which criteria apply when considering inventive step? chanroblesvirtualawlibrary
Answer: In the case of an intermediate used in an inventive process,
the inventiveness may be derived from its contribution to the inventive
process. In the case of an intermediate taking part in an analogy
process (standard process) for the preparation of a patentable
"subsequent product", in order to be inventive the intermediate should
make a structural contribution to the subsequent product and the
structural contribution provided by the intermediate should display ill
least one of those features which differentiate the subsequent product
from known compounds in the prior art. chanroblesvirtualawlibrary
Example 21 Inventive step of a product based on the process for its production chanroblesvirtualawlibrary
Can the inventive step for a chemical product reside in a process for its preparation?
Answer : Only in the case where a technical prejudice to its production
or insurmountable difficulties in its production were believed to
exist. An example is alumina having a high specific surface area (SSA),
used as a catalyst. It would clearly be advantageous to increase the
SSA (increased catalytic activity) but in the state of the art a limit
has been reached. An alumina having a still higher SSA obtained
unexpectedly by a new process is considered to involve an inventive
step. In the claim this alumina must be characterized by its parameters
or as the product of a process.
5.5 Disclosure of invention
Example 22 Chemical compounds-statement of use or effect chanroblesvirtualawlibrary
Has a specific use or effect to be mentioned in an application for a
chemical compound (unless it is obvious for the man skilled in the art
what the use or effect of the compound is)?
Answer: No a specific use or effect for a new chemical compound need
not be stated in order to meet the requirement of industrial
applicability.
N.B.: If compounds having closely related chemical structures are
known, the question arises of how inventive step is established.
Normally, inventive step is considered to be present, if a new chemical
compound has unexpected special properties in comparison with the known
closely structurally-related compounds. If inventive step is considered
with regard only to a structure as a consequence of no reference to use
or effect being present then the requirement of inventive step will
seldom be met. chanroblesvirtualawlibrary
Example 23 Amendment of the disclosure after the filing date chanroblesvirtualawlibrary
May the disclosure of the invention be amended after the filing date of the application by the addition of : chanroblesvirtualawlibrary
(1) an advantage of the invention in relation to the state of the art chanroblesvirtualawlibrary
(2) a property (e.g. melting point, surface tension or
herbicidal properties) of an originally disclosed compound chanroblesvirtualawlibrary
(3) details of the structure of an originally disclosed compound or a corrected formula chanroblesvirtualawlibrary
(4) examples or other technical information? chanroblesvirtualawlibrary
Answer: If it can be shown that the new matter is derived directly and
unambiguously from what is originally disclosed including any features
implicit to a person skilled in the art in what is explicitly contained
in the originally filed application, it may be admitted. Matter which
is derivable directly and unambiguously from the prior art may also be
admitted. If the new matter is not of these two kinds its admission
into the application itself should be refused.
N.B.: Usually subject-matter of the categories (1) to (4) will not be
implicit in the original disclosure and cannot be introduced into the
application. However such subject-matter may be taken into the file and
used as evidence to show inventive step. chanroblesvirtualawlibrary
Example 24 Disclaimers to compounds which cannot be prepared chanroblesvirtualawlibrary
What limitations can be accepted to a claim to compounds of the general
formula N-X (where N is an organic nucleus and X an alkyl group) after
the examiner has objected that certain compounds falling within the
general formula (e.g. the heptyl derivative N-C7 H15) cannot be
prepared? chanroblesvirtualawlibrary
Answer: In order not to be unallowable as new subject-matter, the
limitation must be explicitly or implicitly derivable from the original
application or in the form of a disclaimer. Consequently the limitation
X=Cn (n=7 and above) is acceptable when the examiner is reasonably sure
that the C7 and above compounds cannot be prepared. If C6 is not part
of the original disclosure then the limitation C1 to C6 cannot be
accepted. If, for example, C9 to C12 cannot be prepared then the
limitation would have to be X= Cn (n= 9,10,11,12). However the examiner
should not accept such a limited claim unless he is reasonably sure
that it is concerned with a unitary group of compounds.
5.6 The claims chanroblesvirtualawlibrary
Example 25 Claims containing functional features chanroblesvirtualawlibrary
Is a composition claim allowable if the quantities of substances
forming the composition are defined by a functional feature? For
example, the claim may read as follows, whereby the functional feature,
i.e. the desired result is underlined.
“A selective weed-killing composition comprising herbicidal compound H
1 and herbicidal compound H2 characterized in that the compounds H1 and
H2 are present in quantities that generate a synergistic herbicidal
effect". chanroblesvirtualawlibrary
Answer: chanroblesvirtualawlibrary
A) Claims can be characterized by such a functional feature (i.e. the
obtention of a desired result) only if no other more precise manner of
defining the claimed matter exists. In particular, the result must be
verifiable directly and positively by tests which are adequately
specified in the description. Thus in the above case, if the obtention
of the herbicidal synergistic effect could be adequately and precisely
described by specific ( quantity ratios for the substances H1 and H2,
the above form of claim would not be allowable. chanroblesvirtualawlibrary
B) On the other hand, if there is no more precise manner of defining
the claimed matter and such a functional feature is necessary to ensure
that the applicant obtains adequate protection, the above type of claim
can be allowed provided the conditions of clarity are satisfied. These
conditions are that the skilled man must not only be able to understand
the technical features implied by the wording of the claim but also the
claim must disclose a technical teaching that the skilled man can
perform without unreasonable difficulty. In particular, this means that
those tests which are specified in the description for determining
whether or not the desired result has been achieved must be of a
routine technical nature. chanroblesvirtualawlibrary
Example 26 Definition in claims by parameters chanroblesvirtualawlibrary
Only parameters usual in the art should be employed. This provision
prevents misuse of unusual parameters to disguise lack of novelty.
Definitions using unusual parameters will also make the identification
of products in later search and examination procedure more difficult.
What action should be taken when parameters are used to identify a
chemical product in a claim?
Answer:cralaw:red
A) If the parameters are usual and clear and they are used in such a
way that a comparison with the cited prior art can be made, then normal
examination for novelty is made. chanroblesvirtualawlibrary
B) If the parameters are obscure then the examiner should ask for an explanation of them.
There are then three possibilities: chanroblesvirtualawlibrary
(1) a straightforward explanation possibly with an amendment (e.g. a
simple table of comparisons with usual parameters) is filed which
includes no new subject-matter. This is acceptable. chanroblesvirtualawlibrary
(2) an explanation is filed and/or an amendment is filed which adds new matter that is not allowable. chanroblesvirtualawlibrary
(3) no explanation is filed. chanroblesvirtualawlibrary
In cases (2) and (3), the Examiner should consider refusing the application. chanroblesvirtualawlibrary
C) If the parameters are usual and clear but they are used in such a
way that a comparison with the state of the art is not conclusive, it
might not be feasible to make an objection of lack of clarity since the
application is clear in itself. Where the examiner is of the opinion
that the claimed product is not novel, he should make a tentative
novelty objection asking the applicant to explain what differences
exist between the claimed and prior art products. The examiner can also
ask for a discussion of the prior art. chanroblesvirtualawlibrary
Note: The use of new parameters may, in some cases, be necessary for
the definition of a chemical product and for this reason should not be
prohibited, if convincing arguments are presented. New parameters may
also improve the manner of definition especially if they are used in
addition to usual parameters. chanroblesvirtualawlibrary
Example 27 Analogy processes chanroblesvirtualawlibrary
(1) May several independent analogy process* claims for the
manufacture of a new chemical compound be allowed in the same
application?
Answer: Yes. A combination of analogy process and per se product claims is also allowable.
(2) May several independent analogy process* claims for the manufacture
of a family of new chemical compounds be allowed in the same
application? chanroblesvirtualawlibrary
Answer: Yes, provided the family of chemical compounds is unitary. It
is also possible to allow several analogy process claims, whereby
performance of a claimed process leads to the manufacture of only some
of the compounds of the unitary family, i.e. providing the family of
chemical compounds is unitary the independent analogy process claims
need not have exactly the same scope. chanroblesvirtualawlibrary
(3) Is the following analogy process claim acceptable? chanroblesvirtualawlibrary
"Process for the preparation of compounds according to claim 1,
characterized in that the compounds are prepared by known methods for
the preparation of analogous compounds". chanroblesvirtualawlibrary
Answer: No, the claim must specify, the known methods used in the
claimed process on the basis of information given in the description.
In the absence of such specific indications, the rejection of the claim
would be based upon lack of clarity. chanroblesvirtualawlibrary
* An "analogy" process is a chemical process for the production of a
new chemical compound by which, either (1) the starting materials are
different but of similar constitution to the starting compounds
previously used in the known processes, are reacted together following
the same procedure, to give, as expected new compounds of a similar
constitution to the products obtained by the known processes or (2) the
~ starting materials as used in the known processes are employed in a
similar procedure, to give, as expected new compounds of similar
constitution to the products obtained by the known processes.
Example 28 Independent claims of the same scope chanroblesvirtualawlibrary
May, in an application, several independent claims for the same product
characterized in different ways, using a chemical formula, different
parameters or a product-by-process be allowed?
Answer: Yes, claims defining a product by a chemical formula and
product-by-process claims for the same product are allowable in the
same application. Claims for products defined only by parameters are
also admissible in the same application as other independent product
claims. A product claim is also admissible if it uses a combination of
structural characteristics, process steps and parameters. chanroblesvirtualawlibrary
Example 29 Claims containing generic expressions chanroblesvirtualawlibrary
1. Is the employment of a general expression, such as "alkyl or aryl",
covering a large number of possible substituents, in the following
claim allowable?
chanroblesvirtualawlibrary
" The compound
chanroblesvirtualawlibrary
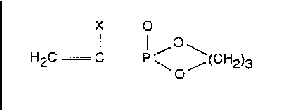
wherein X is selected from hydrogen, halogen, cyano, aryl and alkyl." chanroblesvirtualawlibrary
Answer: Although these expressions are broad they are not necessarily
unclear and their allowability will depend on the circumstances of the
case being examined. There are circumstances when these expressions are
not permissible and objection should be raised. In deciding these
circumstances, the examiner should take into account the teaching of
the prior art and the general knowledge in the art. For example:cralaw:red
(1) The objection can arise that an effect which renders the claimed
compound inventive is not exhibited by all of the claimed compounds. An
objection of lack of inventive step would be appropriate. chanroblesvirtualawlibrary
(2) More rarely the objection might arise that the claimed invention
cannot be carried out over the whole range of the claim. For example,
steric hindrance might render the preparation of some of the claimed
compounds impossible. An objection of lack of sufficiency of the
disclosure should be considered. chanroblesvirtualawlibrary
2. Are claims containing phrases, such as, "possibly substituted aryl
radical" or "the compounds of the above formula may be further
substituted" allowable? chanroblesvirtualawlibrary
Answer: As in case 1 above, although these expressions are broad they
are not necessarily unclear. No objection should be raised in the
following cases:cralaw:red
(1) the description gives a precise definition of the substitution,
allowing the claim to be clearly and easily interpreted. The examiner
should also consider whether it would be appropriate to include this
definition in the claim, or
(2) a clearly defined part of the structure is responsible for the
properties or activity of the compound and the description gives
sufficient indication of the kinds of substituents intended, by
mentioning either their nature or function. However, in this case as
well, the examiner should consider the appropriateness of including an
indication of the kinds of substituents in the claim. chanroblesvirtualawlibrary
In other cases the examiner must decide whether such phrases introduce
obscurity into the claim having regard to what is known from the cited
prior art. Where such a phrase blurs the distinction between the prior
art and the claimed matter it is open to a clarity objection.
5.7 Microbiological processes, genetic manipulation and products obtained by such procedures
Example 30 Novelty of naturally-occurring polymers
A naturally-occurring polymer (e.g. protein, polypeptide, nucleic acid,
polysaccharide) is claimed in an application. For the purposes of this
example, this polymer is referred to as a hypothetical "Protein X". In
the case of nucleic acids and polysaccharides "amino acid sequence"
should be replaced by "base sequence" or "saccharide sequence"
respectively, where appropriate. Is the claim novel in the following
circumstances?
Case 1) Protein X is characterized by its complete amino-acid sequence.
A product having the same amino-acid sequence is disclosed in the prior
art.
Answer: Protein X is not new.
Case 2) Protein X is characterized by its complete amino-acid sequence.
The prior art discloses a product having the same physiological
activity as protein X but does not indi-cate the amino-acid sequence of
the product nor any physico-chemical characteristic or parameter that
enables the examiner to establish whether the prior art product is the
same as or differs from protein X.
Answer: Since several proteins may exhibit similar physiological
activities, the statement of a specific physiological activity in the
prior art is insufficient to unambiguously define the prior art
product, i.e. a specific physiological activity does not imply that the
prior art product and protein A have the same chemical composition and
the same structure. Protein X is new.
Case 3) Protein X is claimed as having a specific physiological
activity and is characterized by physico-chemical characteristics. The
prior art discloses a product having the same physiological activity as
Protein X and certain other physico-chemical characteristics.
Answer: To establish whether protein X and the prior art product are
the same the examiner can only employ characteristics which
unambiguously identify a single protein. These characteristics do not
include molecular weights and isoelectric points as they are not unique
characteristics of a single protein. The instructions with regard to
characterizing products by parameters are to be followed i.e. a
tentative objection of lacking novelty must be raised in specific
circumstances. The examiner must ensure that protein X, which is
identified by certain parameters is not a mere re-writing of a product
that is already known and which is identified by other parameters.
Case 4) The prior art discloses a protein X but only in the form of an
impure mixture. The application claims a purer form of protein X.
Answer: The criteria for selection inventions are to be employed in
determining whether or not the purer product is novel. The prior art is
considered to relate to a composition of the protein X and impurities
whereas the purer product is a selection within this composition
containing a smaller amount of impurities. It follows that the degree
of impurity of the claimed product must be substantially below that
encountered in the prior art and claimed purer product must achieve a
specific purpose or specific technical effect which differs from that
of the prior art (purposive selection). If these criteria are fulfilled
then the purer claimed product is novel.
Case 5) Protein X is known in a form which is pure from other
contaminating proteins but the amino-acid sequence of protein X was not
disclosed. An applicant claims the amino acid sequence of protein X.
Answer: A claim directed to the amino-acid sequence of a known protein lacks novelty.
Case 6) Protein X is known but the applicant has obtained it for the
first time using recombinant DNA technology. The applicant claims the
protein A so obtained. Answer: Such a claim lacks novelty, as to
obtain a known protein using a different technology does not confer
novelty on the resultant product, however difficult or time- consuming
the technology might be.
Case 7) Protein X was known in a glycosylated form. It is now claimed
"unglycosylated". Answer: The unglycosylated protein is novel. Protein
X with a different glycosylation pattern than that disclosed in the
prior art would also be novel.
Example 31 Novelty-destroying effect of micro-organisms mentioned in prior art
What is the novelty-destroying effect of micro-organisms cited in the
prior art, e.g. in patents or in scientific publications. but which are
neither commercially available nor obtainable from a depository?
Answer: For the sake of defining the novelty-destroying effect of such
micro-organisms, it is assumed, unless the contrary is established,
that these micro-organisms are available as of the date of publication.
In fact many scientific periodicals request from the authors whose
articles they publish, that samples of the biological material cited in
the articles be delivered to third parties on request.
In the same way. when a patent or patent application relies upon a
previously published document for sufficiency of disclosure, if this
previously published document uses a micro-organism, it is assumed that
the micro-organism is available even in the absence of a deposit.
Example 32 Patentability of claimed DNA sequences
Question 1: Is the following claim allowable? :cralaw:red
"DNA sequence coding for Protein A" where "Protein A" meets the requirements set out in the cases indicated below?
Case 1 : "Protein A" is a novel inventive protein.
Answer: In such a case, providing the DNA sequence itself is new, the
broad claimindicated above is acceptable. The claim may cover a
specific sequence as well as all sequences coding for "Protein A" due
to the degeneracy of the genetic code.
Case 2 : "Protein A" is known, but its chemical structure is not.
Answer: If it was possible for the man skilled in the art to sequence
"Protein A" at the date of filing of the application, then there would
be no inventive step in deducing the DNA sequences which would code for
the protein. Thus the above claim would lack inventive step.
Case 3 : "Protein A" is known by its amino-acid sequence.
Answer: The skilled man can derive the DNA sequences coding for "Protein A" and therefore the above claim lacks inventive step.
Question 2:: Can a claim relating to the selection of a specific DNA sequence coding for a protein be allowed?
Answer: Yes, since an invention may reside in the choice of a specific
DNA-sequence, selected in a non-obvious way from a pool of all possible
sequences which would code for the protein such that an unexpected
result or effect could be achieved. The unexpected effect might be, for
example, the expression of the desired protein in a specific host cell,
or the use of a specific host/recombinant vector system.
Example 33 Claims to recombinant vectors
How is a recombinant vector, e.g. a plasmid, to be characterized?
Answer: A recombinant vector, being a chemical product, may be characterized:cralaw:red
(a) by its formula (DNA sequence)
(b) as the product of a process
(c) by a combination of parameters and/or properties (cleavage map, molecular weight, number of base pairs, etc. )
(d) by the composition of its sub-parts (origin, markers, promotors, replicons, etc. …)
It may also be characterized by reference to a deposit of a biological material in which the recombinant vector is present.
Applicants often use their own private and arbitrary designation to
characterize therecombinant vector in the description and claims. Such
a designation is meaningless for third parties and cannot be accepted
as clearly identifying the product.
Examcle 34 Hybridomas and monoclonal antibodies
Is the following set of claims acceptable where the prior art is defined as indicated in the cases A and B below?
1. Monoclonal antibodies against K
2. Hybridoma cell line producing monoclonal antibodies according to claim 1.
3. Process for the preparation of a hybridoma cell line according to
claim 2 characterized in that spleen cells derived from a mouse which
has been previously immunized with K are fused by conventional
techniques, with mouse myeloma cells and that subsequent selection of
the hybrid cells producing the desired antibodies takes place.
Case A -Prior Art :cralaw:red
K is known as an antigen, or it is readily derivable for the man
skilled in the art that it has immunogenic properties (e.g. K is a
protein). Monoclonal antibodies against the K antigen have not yet been
prepared.
Case A -Answer:cralaw:red
It is well-known that by use of the classical fusion technique of
Kohler and Milstein (Nature 1975, volume 256, page 495), monoclonal
antibodies against a predetermined antigen can be routinely obtained so
that claim 1 is not acceptable for lack of inventive step.
On the other hand, claims directed to specific hybridomas (which
generally must be deposited) and to monoclonal antibodies produced
thereby which display unexpected properties or effects (e.g. a low
cross-reactivity with other antigens) are considered to involve an
inventive step. In such a case the claims should be drafted in terms of
the technical features which characterize the invention, e.g.
"Hybridoma cell line having the deposit accession number ATCC TIB 148"
"Monoclonal antibodies against the K antigen obtainable from the
hybridoma cell line having the deposit accession number ATCC TIB 148"
Case B -Prior Art :cralaw:red
K is unknown or its immunogenic properties are not readily derivable for the man skilled in the art.
Case B -Answer:cralaw:red
Claims directed generically to hybridomas producing monoclonal
antibodies against the antigen, to the monoclonal antibodies per se and
to the method of preparation of the hybridoma are acceptable. No
deposition of any particular hybridoma is required, unless some
specific hybridoma are claimed, because it is considered that by use of
the classical fusion technique of Kohler and Milstein (See claim 3)
monoclonal antibodies of generic specificity can be routinely produced.
In this case the patentability of the hybridomas and of the monoclonal
antibodies is assessed at the level of the antigen, which needs to be
clearly defined in the specification so that its preparation is
reproducible. In this case, provided K is novel, a product claim
directed to the antigen K could also be acceptable.
Example 35 Claims for biological materials per se
How should a claim to a new biological material be formulated?
Answer: There are four possibilities:cralaw:red
(1) Where a strain of micro-organisms or another biological material
has been deposited with a recognized depository institution, the best
way of defining the biological material in a claim will be to use the
file number of the deposit and the name of the institution. Preferably,
the name of the genus and species, to which the strain belongs should
also be quoted, although this is not obligatory. Thus such a claim
could read:cralaw:red
"Biological material of the (genus) (species) (depositary institution) (file number of the deposit)."
(2) The biological material can be described by the process used to
obtain it from a known biological material. Only when the process used
is repeatable is it possible to use a "product-by-process" claim (See
NB.1 below).
(3) The biological material can be described by means of parameters,
in particular functional parameters, such as resistance to growth
inhibition. An example of such a claim could read:cralaw:red
"An L-histidine producing biological material belonging to the genus X
characterized by a resistance to growth inhibition by RNA polymerase
inhibitor."
-assuming that the growth of biological material of genus X is
otherwise inhibited by RNA polymerase inhibitor, the characterizing
feature of the above claim constitutes a simple routine test for
distinguishing the claimed biological material from those of the prior
art.
(4) The ability of a micro-organism to produce or transform an
organic compound can be used to characterize a micro-organism, See
above, Chapter II, 6, Annex 1, point 2.2).
Note: Processes for the isolation of biological material from natural
sources, e.g. soil samples, and for obtaining biological material by
natural mutation are not generally repeatable. Artificial mutation
processes mayor may not be repeatable whereas genetic engineering
processes are often repeatable.
Example 36 Microbiological inventions
Assuming that a deposit of a claimed biological material is necessary,
will it be necessary to deposit several members of a "group" of
biological material indicated in the claim, e.g. several mutants of a
strain? Answer: The examiner should not decide that the number of
deposits is insufficient to support a claim unless he has in his
possession a document showing this or alternatively if he is sure.
Example 37 Generalizations in the field of microbiological inventions
Can biological material be protected by product claims covering a genus
or a species when only a few strains or even a single one is/are
accessible?
Answer: Yes, this is in principle possible. It is however highly
unlikely that such a claim could be sufficiently supported by the
description in the case of generalization to a genus. In order for the
scope of claims to be clear, an acceptable generalization could be such
that all the biological material covered have a common property or
activity and the description gives sufficient taxonomic information to
identify the biological material which are covered by the claims.
Example 38 Terminology in claims for biological materials
Question 1:cralaw:red
Are the terms "mutant" and "variant" permissible in claims for biological material per se?
Answer: The use of such terms generally raises a problem of lack of
clarity. Up to now there is no internationally recognized definition of
what "variant" exactly means, so the examiner has to carefully consider
case by case on the basis of the description, whether or not the scope
of protection is clear. Since the obtention of mutants is always based
on a random process the term "mutant" may also be a source of
unclarity. Unless the description defines these terms differently, they
should be given their ordinary and customary meanings which are the
following:cralaw:red
Mutant -any organism which differs from the parent strain by
modification in the genotype as the result of one or more mutations.
Variant -a strain which differs in some (often minor) way from a particular, named organism.
A possible way to make clear the scope of protection is to introduce a
functional limitation, such as a limitation to those mutants and
variants of a specific strain which perform the same function as the
parent strain. "Xanthomonas compestris NRRL B 1459 and mutants and
variants thereof capable of producing the polysaccharide of claim x".
Question 2:cralaw:red
If a claim is directed to a parent strain of a biological material and
its mutants and the scope of protection provided by the claim is clear,
is it necessary to deposit one or more of the mutants?
Answer: No, provided that .the parent strain is available, the mutation
technique is taught and there is evidence of acceptable mutation rates
leading to stable mutants, deposits of the resultant mutants are not
required for sufficiency of disclosure.
Question 3:cralaw:red
Is a deposit needed for a claim to a specific mutant or variant?
e.g. "Mutant Y-1 of Streptococcus NRRL 234567"
Answer: Generally, yes because it is very unlikely that such a mutant
can be obtained reproducibly from the parent strain. Mutational
techniques which make use of mutagenic agents (physical or chemical
agents) are random techniques based on trial-and-error and, therefore,
it is highly unlikely that, starting from an available micro-organism,
a specific, unique mutant can be reproducibly obtained. On the other
hand, it should be borne in mind that, in the era of gene technology,
mutational techniques may be developed which allow the precise and
reproducible introduction of a specific mutation into the genome. In
such a case a specific, unique mutant would also be reproducible in the
absence of a deposit.
Example 39 Novelty of biological material
How does the examiner, establish, whether, or not biological materials,
which. at least ,Seem to be very similar to biological material
mentioned In a prior art document (which possibly may refer to a
deposition), are novel?
Answer: If the examiner cannot recognize clear, distinguishing features
he should in the case of the search examiner, cite the prior art
document In the search report and, In the case of the substantive
examiner, raise a novelty objection. The applicant will then have to
show sound reasons why the alleged identity is not present and, if he
fails to show this, the Examiner should refuse the application.
Example 40 Inventive step for biological material
A new biological material is claimed per se, the form of claim being:cralaw:red
"Streptomyces P. NRRL 123456." It is disclosed in the application that
this biological material is capable of producing a Specific compound X.
Is it possible to accord inventiveness to the biological material
automatically, bearing in mind that it was impossible to predict that
the claimed biological material would be capable of forming the new
compound X?
Answer: No, an automatic admission of inventiveness is not generally
possible. The prior art relating to the compound X must be considered.
The biological material can be considered inventive either
(A) when the compound is itself inventive, for example:cralaw:red
(i) if X exhibits unexpected effects or advantages with respect to close compounds of the prior art, or
(ii) if the compound X is structurally not close to compounds of the
prior art, so that its properties could not have been predicted.
(in both (i) and (ii) above the biological material is the means by
which the unexpected effects or advantages of compound X are obtained)
or
(B) when the biological material enables the performance of an inventive process, for example:cralaw:red
(iii) if X is structurally close to known compounds and no unexpected
effect is present, but the choice of the biological material is in
itself unexpected for the manufacture of compound X, e.g. when the
prior art is concerned with a different genus for preparing the
structurally close compound and the examined case is the first one in
which it is disclosed to use a strain of streptomyces for the
preparation of this type of compound. Lack of inventiveness of the
biological material should be objected to in the case where X is ;
structurally close to known compounds and no unexpected effect is
present (as in the case (iii) above) and furthermore, the new
biological material is a mutant of a strain known to be capable of
making compounds structurally close to X and having the same utility.
Then it can be argued that it is obvious for the skilled man to mutate
the known biological material to obtain a new strain capable of making
the new compound X.
Note: Although inventive step for a biological material can normally
be assessed with reference to a compound which it produces other
properties of, biological material can sometimes be used for the same
purpose, e.g. the ability to metabolize a noxious pollutant.
Example 41 Biological material -availability to the public
The applicant has three options if he claims a microbiological process
or the product thereof which involves the use of a biological material
not apparently available to the public. He can, in order to disclose
the invention sufficiently:cralaw:red
(i) show that it is available, or if it is not available he can,
(ii) describe it in such a manner as to enable the invention to be carried out by the person skilled in the art, or
(iii) he can deposit a culture of the biological material.
For the examiner the question is not whether the biological material
can be described, but whether it is described so that the disclosure is
sufficiently clear and complete.
Question 1:cralaw:red
Should the examiner consider a biological material to be available to the public in the following instances?
(a) The applicant alleges that the biological material is available (without further information).
Answer: No, unless the biological material is known to be readily
available or known to be deposited with a depository institution and
available from there at the date of filing.
(b) The applicant says nothing about the availability of the biological material or its deposit in a depositary institution.
Answer: No, as under (a).
Question 2:cralaw:red
What measures must the examiner take to ensure that a biological
material is available to the public when a biological material has been
identified by a deposit, so that it is certain that the skilled man can
carry out the claimed invention and the requirements of sufficiency are
met?
Answer: Where the applicant and depositor are the same and the deposit
has been made with a recognized depository, it can be assumed that the
conditions under which the deposit has been made are such that the
biological material is publicly available. If the applicant and
depositor differ then the examiner should check with the depository
"that the biological material is available to the public. Exceptionally
a subsidiary (as applicant) which is controlled by a parent company (as
depositor) can be considered as a single entity so that a check is
unnecessary in these circumstances.
Example 42 Characterization of deposited biological material
In the case of inventions concerning microbiological processes or the
products thereof and involving the use of a biological material "that
the application as filed should give relevant information as is
available to the applicant on the characteristics of the biological
material".
What consideration should the examiner give to this information?
Answer: He should assume that it is correct and sufficient, any doubts
being resolved in favor of the applicant. However requests to add to
the description further information on the characteristics of the
biological material should be refused as new subject-matter. Such
information may remain in the file and be considered by the examiner
when necessary. On the other hand, the name of the depositary
institution and the file number of the culture are to be entered into
the application within 16 months of the date of filing. The inclusion
of this information is not new subject-matter and is necessary to
fulfill the requirements of sufficiency.
Example 43 Priority provisions for microbiological inventions
(a) What particular conditions have to be fulfilled with regard to priority in microbiological inventions?
Answer: In the case of inventions referring to micro-organisms not
available to the public at the date of filing of the first application
(irrespective of whether they have become available or not at the date
of filing the application), priority should be recognized in all cases
in which the applicant can show reasonable evidence, that the
micro-organism referred to in the application is identical with the
micro-organism referred to in the first application. Such evidence can
be
- based on a deposit at the date of filing the first application,
- with a recognized culture collection (Budapest Treaty)
- with a culture collection recognized by any national office
-
with any culture collection not recognized, provided there is no
evidence against the sound basis of that collection
- or other evidence as there may be according to the case,
bearing in mind that statements of the applicant should only be doubted
for specific reasons.
(b) Should priority be accepted if the applicant has,
simultaneously with original filing, deposited the micro-organism with
a culture collection that is not recognized by the Budapest treaty and
later deposits the micro-organism with a .recognized culture collection
and files an application?
Answer: Yes, according to the provisions in the answer under (a), provided the identity of the two deposits is proved.
(c) Should priority be accepted if the applicant has filed his
original application without any deposition of the micro-organism, in a
country where deposition is not required and the micro-organism is not
available to the public and where later, the applicant deposits the
micro-organism with a recognized culture collection and files an
application?
Answer: Yes, according to the last paragraph of the answer to (a), provide the micro-
organism with which the priority application is concerned and the micro-organism which is deposited are proved to be identical.
|
